NCERT solution for class 10th // Science // Chapter 4 -Carbon and its compound
NCERT solution > Science > Class 10th > Chapter 4 -Carbon and its compound
NCERT solution for class 10th // Science // Chapter 4 -Carbon and its compound
Page number 61
Q1. What would be the electron dot structure of carbon dioxide which has the formula CO2
Answer .
Electron dot structure of CO2 is
Q2. What would be the electron dot structure of molecules of sulphur which is made up of eight atoms of sulphur ( Hint- the 8 atoms of sulphur are joined together in the form of a ring)
Answer
Page number 68
Q3. How many structural isomers can you draw for pentane
Answer
Pentane has a five carbon atoms which can be arranged in straight chain or in the form of branch the molecular formula of pentane is C5 h12 it has following isomers
Q4. What are the two properties of carbon which lead to the use number of carbon compounds we see around us
Answer
The two main properties of carbon which lead to the form of huge number of carbon compounds are
- Tetravalency of carbon
- Catenation
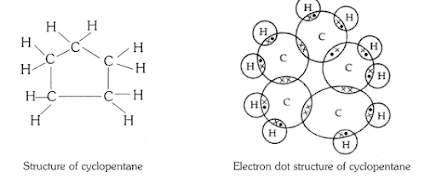
Q5 . What will be the formula and electron dot structure of cyclopentane
Answer
Cyclopentane has five carbon atom the general formula of alkene is CnH2n general formula of cyclopentane is C5 H10
The electron dot structure of cyclopentane
Q 6 . Draw the structures for the following compounds
- Ethanoic acid
- Bromo pentane
- Butanone
- Hexanal
Are structural isomers possible for bromopentane
Answer
Q7 . How would you name the following compounds
Answer
a) It is bromo ethane because it has two carbon
b) It is maternal because it has a single carbon with the functional group C= O
c) It is 1 hexyne because it has six carbon compound and the triple bond at the first position
Page number 71
Q8 . Why is the conversion of ethanol to ethanoic acid an oxidation reaction
Answer
Oxidation reaction is nothing but the addition of Oxygen and the removal of hydrogen atom from any compound and during the conversion of ethanol to ethanoic acid the two hydrogens remove and one oxygen is added so it is oxidation reaction
Q9 . A mixture of Oxygen and ethyne is burnt for welding can you tell why a mixture of ethyne and air is not used ?
Answer
For the welding high temperature is required but when ethyne is burnt in air it undergoing incomplete combustion and release the lot of smoke and and temperature is not very high so avoid that situation .The mixture of ethyne and oxygen is used
2CH
= CH + 5O2→4CO2 +
2H2O + Heat + Light
Page number 74 .
Q10. How would you distinguish experimentally between an alcohol and carboxylic acid
Answer
Alcohol and carboxylic acid can be distinguished by following reaction .
Reaction with Sodium bicarbonate .
When carboxylic acid react with sodium carbonate it gives bricks effervescence of CO2 gas while alcohol do not react with it
CH3COOH+ NaOH → CH3COONa + H2O
CH3CH2OH+
NaOH → No reaction
Reaction with base
Carboxylic acid react with base to form salt and water while alcohol do not react with base
CH3COOH+ NaHCO3 → CH3COONa + H2O + CO2
CH3CH2OH+ NaHCO3 → No reaction
Q11. What are oxidising agents ?
Answer
The substance that are capable to providing the oxygen to other substance are called oxidizing agent
Example - Potassium per magnet ( KMnO4)
and Potassium dichromate ( K2Cr2O7)
Page number 76
Q12. Would you be able to check if water is hard by using a detergent ?
Answer
No we are unable to check if the water is hard by using a detergent because does a detergent does not form a foam with hard water
Q 13. People use a variety of methods to wash clothes usually after adding the soap they beat the clothes on the stone or beat it with a paddle scrub with a brush or the mixture is agitated in a washing machine why is agitation necessary to get clean clothes ?
Answer.
Clothes are soaked in a soap solution then the dirt form a micelles with the soap solution and to loosen the dust particle in the form of micelles it is necessary to scrapped the cloth or beaten or agitated in washing machine
Exercise
Q1. Methane with a molecular formula C2 H6 has
a) 6 covalent bonds
b) 7 covalent bonds
c) 8 covalent bonds
d) 9 covalent bonds
Answer - 7 covalent bond
Q2. Butanone is a four carbon compound with the functional group
a) Carboxylic acid
b) Aldehyde
c) Ketone
d) Alcohol
Answer - Ketone
Q3. While cooking if the bottom of the vessel is getting blackened on the outside it means that
a) The food is not cook completely
b) The fuel is not burning completely
c) The fuel is wet
d) The fuel is burning completely
Answer - The fuel is not burning completely
Q4. Explain the nature of covalent bond using the bond formation in CH3Cl
Answer
A CH3Cl is a chloromethane which contain one carbon atom three hydrogen atom and one chlorine atom all these are bounded by covalent bond this bond formed by the sharing of electron because carbon has four electrons in its valence shell and to complete its octet .It needs to gain the four electron or to lose the four electron but it is quite impossible so that carbon atom form a bond with other atom by sharing of electron and this bond is known as covalent bond
Electronic configuration of carbon ( 6) = -(2 ,4)
Electronic configuration of hydrogen (1)= 1
Electronic configuration of chlorine ( 17)= (2,8,7)
Q5 . Electron dot structure for
a) Ethanoic acid
b) H2S
c) Propanone
d) F2
Answer
Q6. What is an homologous series explain with an example
Answer
A Series of a compound in which the same functional group substitute and same chemical properties of two successive members of series is called homologous series
Example - alkane series CnH2n
+ 2
- CH4 - Methane
- C2 H6 - Ethane
- C3H8- Propane
- C4 H10 - Butane
- C5 H12 - Pentane
Q7. How can ethanol and ethanoic acid be differentiated on the basis of their physical and chemical properties
Answer
On the basis of physical properties
- Smell of the ethanoic acid has pungent while ethanol has pleasant smell
- Ethanoic acid is solid while ethanol is liquid at room temperature
- Melting point of ethanol is low 156 K while the ethanoic acid is high 290 k
On the basis of chemical properties
- Ethanoic acid react with bases like anywhere and queue used to form salt and water while ethanol does not react with them
- CH3COOH + NaOH. → CH3COONa + H2O
- CH3COOH+KOH → CH3COOK+H2O
Q8. Why does micelle formation take place when soap is added to water ? will a micelle be formed in other solvents such as ethanol also ?
Answer .
Green soap added to water at the surface of water the hydrophobic and or tail of the soap will be in soluble in water and the soap being and now with the surface of water with the ionic and inverter and the hydrocarbon producing out of water
Q9 . Why are carbon and its compound used as a fuel for most applications
Answer
Carbon burn in air produced carbon dioxide and water , during this reaction large amount of heat and light energy is released . so, they are used as a fuel .
C + O2 → CO2 + Heat+ Light
Q10. Explain the formation of scum when hard water is treated with soap
Answer
Soap contains Sodium and potassium salt while hard water contains calcium and magnesium ion when soap is added to hard water then calcium and magnesium salts are formed these are insoluble in water and get precipitated .This precipitates are called scum
Reaction shown by scum
Ca 2+ + 2 R COONa. → (RCOO)2 Ca + 2 Na +
Hard water + soap → Calcium salt precipitate
Mg2+ + 2 R COONa. → (RCOO)2 Mg + 2 Na +
Hard water + soap → Magnesium salt precipitate
Q11. What change will you observe if you test soap with litmus paper ( red and blue)
Answer
Soap turns red litmus paper into blue and have no effect on the blue litmus paper because soap is alkaline in nature
Q12. What is hydrogenation what is its industrial application
Answer
The addition of hydrogen to unsaturated hydrocarbons in the presence of catalyst is known as hydrogenation
Industrial application
When hydrogen gas is passed through vegetable oil in presence of nickel catalyst it changes to solid fat (ghee)
Vegetable oil + H2. → fat ( ghee)
Q13 Which of the following hydrocarbon undergo addition
C2 H6 ,C3 H8 ,C3
H6 ,C2 H2 and CH4
Answer
Unsaturated hydrocarbon like alkenes and alkynes containing double and triple bond so they are undergoing addition reaction
C3 H6 , C2 H2 , will undergo in addition
reaction
Q14. Give a test that can be used to differentiate between saturated and unsaturated hydrocarbons
Answer
Butter contain saturated compounds while cooking oil contain unsaturated compounds when unsaturated compounds are oxidized by alkaline KMnO4 it disappears its pink colour when cooking oil is treated with alkaline KMnO4 pink colour of KMnO4disappears but with butter the pink colour of KMnO4does not disappear
Q15. Explain the mechanism of the cleaning action of soaps
Answer
Soap molecules have different properties at their two ends hydrophilic and hydrophobic at the surface of water the hydrophilic end it will be in soluble in water and the soap in a lying along the surface of water with the ionic and in water of hydrocarbon tail protruding out of water
Inside the water the molecule shows the similar orientation that gives the hydrocarbon portion out of the water this form a cluster of molecules is called micelle
It is easily to clean out from water











Comments
Post a Comment
Text